In face of the current Covid-19 pandemic, the Infectious Diseases Data Observatory (IDDO) has been developing several Medical Product Quality Reports about substandard and falsified (SF) COVID-19 medical products and COVID-19 vaccines, which include data from scientific literature, public alerts and warnings, and lay press from across the world.
These monthly reports aim to collate information and reports in the public domain on the quality of medicinal products that are currently in use, or that are being trialled for COVID-19’s prevention or treatment. IDDO currently issues two different monthly reports in response to the COVID-19 pandemic to highlight the risk of substandard and falsified products: Medical Product Quality Reports on COVID-19 vaccine issues and Monthly Medical Product Quality Reports on COVID-19 supplies.
LATEST REPORTS
Medical Product Quality Reports - COVID-19 vaccines
Issue 13 - Data from August & September 2021
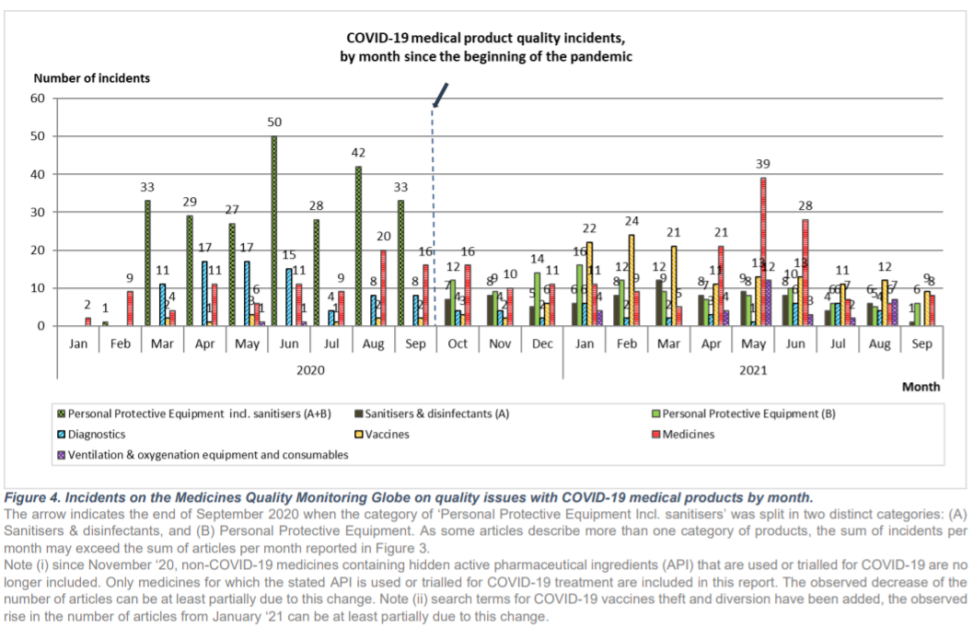
The COVID-19 pandemic is accompanied by a multitude of reports of diverted, substandard and falsified (SF) medical products. Since the beginning of the pandemic, IDDO’s report on COVID-19 issues has identified 906 relevant articles on quality problems with COVID-19 medical products in the English language lay press.
The latest issue, covering the months of August & September 2021, reports on 64 different incidents of quality issues with COVID-19 medical products from articles from searches in the English language Google News.
In the first part of the report, we can see a further summary of public domain reports on quality issues with COVID-19 vaccines. So far, IDDO’s Medical Product Quality Report has been recorded 172 reports of incidents on quality issues with COVID-19 vaccines linked to 44 different countries. In August and September 2021, 22 new incidents were reported. For the first time, quality issues were found in Turkey, Spain, and Zambia. Eight incidents involved falsified COVID-19 vaccines including products labeled as Pfizer/BioNTech, Covishield, and Johnson & Johnson. Several articles reported on apparently contaminated Moderna COVID-19 vaccines in multiple prefectures in Japan.
The second part of the report discusses diagnostics, Personal Protective Equipment (PPE), sanitisers & disinfectants, medicines, and ventilation & oxygenation equipment and consumables. For PPE, masks remain the main affected product. Some cases of poor quality remdesivir and tocilizumab occurred. In Thailand shortly after approval of green chiretta, a herbal medicine to treat asymptomatic COVID-19, a falsified version was detected.
UPDATE
Medical Product Quality Reports – COVID-19 issues
Issue 11- Data from April & May 2021

The 11th issue of the report shows that 135 new public domain incidents on diverted or substandard or falsified COVID-19 products identified on the Medicines Quality Monitoring (MQM) Globe were reported. Sadly, a few healthcare professionals have been involved in diversion or falsification of COVID-19 medicines.
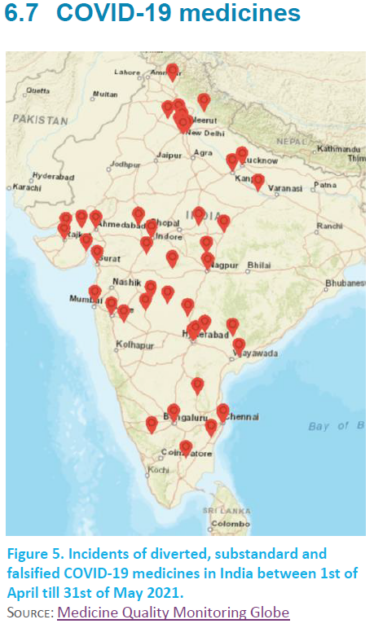
Almost two-thirds of incidents in this issue were reported in India as the demand for COVID-19 supplies drastically exceeded supply during the second wave the country experienced. Regarding the medicines which were most reported, remdesivir, amphotericin B, and tocilizumab are the main ones. 88.3% of the incidents were reported for remdesivir. For more information on falsified COVID-19 medicines see page 30.
This report also highlights a surge of diverted or SF ventilation and oxygenation equipment and consumables. Conversely, the reported incidents relating to other COVID-19 medical products such as personal protective equipment, sanitisers & disinfectants and diagnostics seem to have decreased.
Medical Product Quality Reports – COVID-19 vaccines
Issue 5 – June 2021 (Data up to 31 May 2021)

The newest update of this cumulative report includes information identified up to 30th April 2021 (changes are indicated in red).
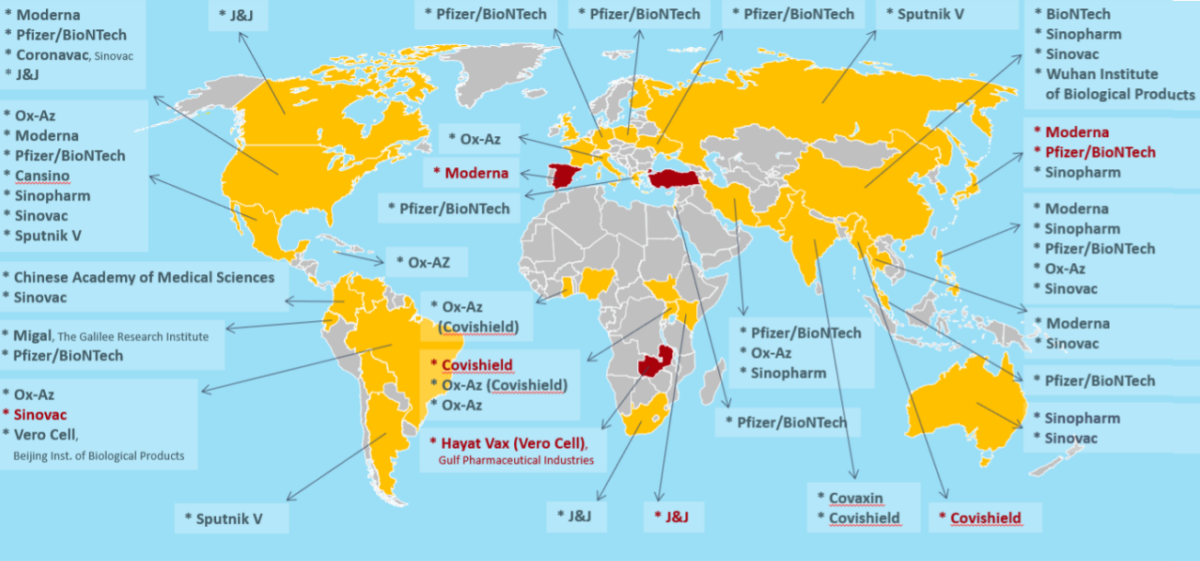
Between 12th March 2020 and 31st May 2021 IDDO has found 123 reports of diverted or SF COVID-19 vaccines from 35 countries in the press. Out of all unauthorised copies of approved vaccines, authorities seized copies of products from Pfizer/BioNTech (15), AstraZeneca (7), Sinovac (5), Sinopharm (5), Moderna (5), Sputnik V (4), and Johnson & Johnson (1), which were reported online from 12 countries (Australia, Brazil, China, Germany, Italy, Japan, Malaysia, Mexico, Philippines, Poland, USA, Ukraine). Germany and Poland were the latest countries to seize these SF products. In addition, Sputnik V, is the newest addition to the list of online selling unauthorised copies of approved vaccines.
Although during the last two months there have been fewer press articles reporting on incidents with diverted or SF COVID-19 vaccines, during the first quarter of the year there were over 20 different reports per month. This was an exponential increase compared to the number of incidents in 2020.
During April and May 2021 IDDO documented 14 reports per month and almost half of them were related to diverted and/or unregistered COVID-19 vaccines. Nevertheless, IDDO explains that further analysis is needed to better understand the recent decrease in incidents and to determine if there are fewer incidents in recent months or if the phenomena of media fatigue is at play.
Previous FTF report posts:
Medical Product Quality Report on COVID-19 vaccines
Medical Product Quality Reports on COVID-19 supplies