INFORMATIONAL MATERIALS
Fighting the fakes: tackling substandard and falsified medicines
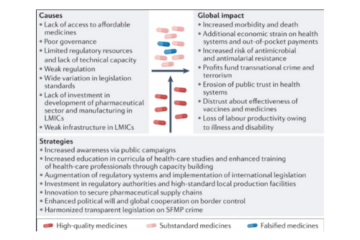
A recent comment written by Oksana Zirka Pyzik (UCL Fight the Fakes academic chapter founder and lead) published by nature reviews highlights how poor-quality or fraudulent medicines are causing extensive humanitarian and economic harm, particularly in Africa. Authors claim these products are a global problem that requires a coordinated cross-border response, including enhanced Read more…