This press release was originally published on Alliance for Safe Online Pharmacies.
Fake Medicines Continue to Threaten Consumers – EU Member States have an Obligation to Act
FOR IMMEDIATE RELEASE
21 February 2018
According to the Alliance of Safe Online Pharmacy in the EU (ASOP EU), the illegal manufacturing and distribution of fake medicines is an enormous and growing public health risk with an untold cost to lives. Vast profits are made by organized criminal gangs who often use the proceeds to support other criminal activities.
The Internet provides the ideal channel to buy illegally distributed fake medicines. Price, convenience and secrecy have driven consumer demand, creating the perfect environment for over 35,000 illegally operating websites.
A major part of EU regulation comes into effect early next year, putting in place safety features on every single pack for prescription medicines in the EU. These comprise a unique bar code and tamper-proof packaging, which enables verification at the point of dispensing. This will undoubtedly tighten security around the traditional routes of medicines supply but it does not tackle the problem of illegally distributed fake medicines on the Internet.
Under article 85d of the Falsified Medicines Directive, each EU Member State is obliged to enable legal sellers of medicines (pharmacies and retailers of medicines) to sell their medicines on the Internet. This is achieved through the implementation of a mandatory common logo on each and every website page, which links through to an official state registry. All legal sellers of medicines must register with the Competent Authority in EU Member States where their business is operating.
Public facing campaigns to raise awareness about the risks of buying medicines online are also a legal obligation for EU Member States and the EU Commission for Health and Food Safety under the Falsified Medicines Directive.
To better understand how countries are working to educate and protect consumers, the European Union’s Alliance for Safe Online Pharmacy (ASOP EU) and the European Alliance for Access to Safe Medicines (EAASM), has convened a series of discussions with many Member States. Based on these meetings, ASOP EU and EAASM have today issued a report, “FIGHTING FAKES BY MEMBER STATES – Falsified Medicines and the Common Logo – Raising Public Awareness.” The report provides a detailed snapshot of the progress each EU Member State has made to educate the public about the risks of buying medicines on the Internet.
“We applaud our partners’ commitment to raising awareness about the dangers of buying medicines online. However, it is clear that more activity by Member States, with their own campaigns is needed to build on the many initiatives across the EU that combat this growing threat to public health,” said Mike Isles, Executive Director of ASOP EU.
The infogram below on the status of these campaigns indicates that efforts are highly variable by country and organisation
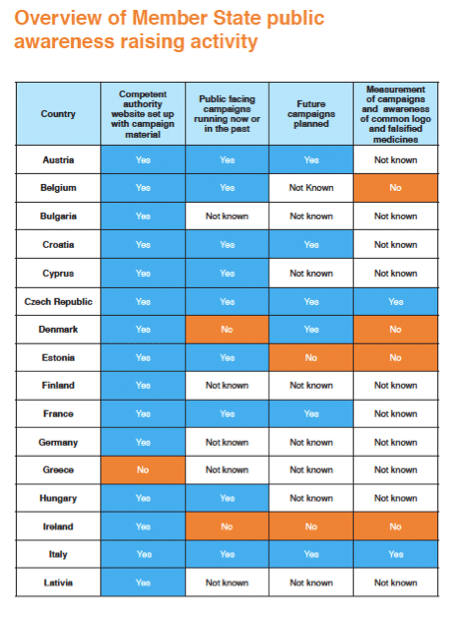
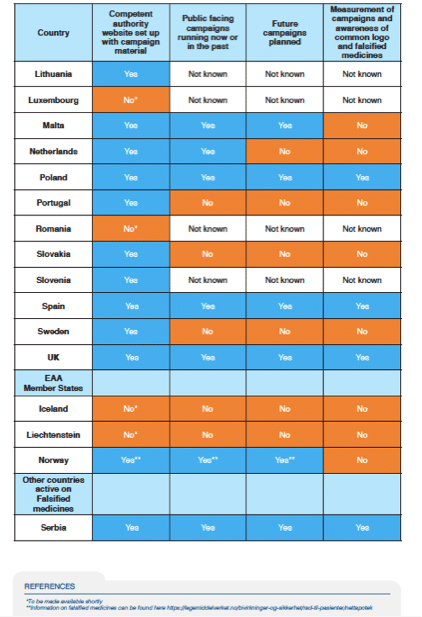
The report highlights a number of effective awareness initiatives by Member States. An exemplary example is one by Medicines and Healthcare Products and Regulatory Agency’s (MHRA) ‘FakeMeds#’ social media campaign.
“Medicines, by their very nature, are not ordinary consumer goods and their sale and supply is strictly controlled. Prescription only medicines (POMs) in particular are potent and have the ability to harm, as well as cure; consequently, they should be prescribed by a health care professional and dispensed through a registered pharmacy. Websites offering to supply POMs without a prescription are not only breaking the law but are putting patients’ health in jeopardy. The criminals behind these sites are not interested in people’s health and well-being – only their money. Many sites supply medicines that are not authorized and therefore their safety and quality has not been tested. Our advice is “Don’t gamble with your health” – if you have a concern about your health – speak to your doctor. The MHRA supports ASOP in their work with European regulators and driving forward crucial public health messaging” said Lynda Scammell, Senior Policy Adviser at the MHRA’s Enforcement Group.